Review Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Research Progress on The Related Mechanism of Diabetic Angiopathy and TCM Collateral Diseases Treatment Scheme
*Corresponding author: Da peng Wang, Hospital of Dalian Medical University, Dalian, China.
Received: August 18, 2022; Published: October 13, 2022
DOI: 10.34297/AJBSR.2022.17.002332
Abstract
The two most common types of diabetic complications include macrovascular disease and microvascular disease, among them, diabetic nephropathy (DN) is one of the common clinical diabetic microvascular complications and an important cause of endstage renal failure in diabetic patients, which has become the main cause of death. The pathogenesis of diabetic nephropathy is very complex and is known to be related to a variety of factors, including hemodynamic changes, oxidative stress, genetic factors, inflammatory mediators, impaired glucose metabolism and cytokines. Although strict control of blood glucose can delay the progression of the disease, the current treatment effect is still not ideal. In recent years, TCM collateral diseases have played an important role in the treatment. This paper reviews the research progress of vascular diseases and the treatment of TCM collateral diseases.
Keywords: Diabetic Nephropathy; Pathogenesis; Microcirculation Disorder; Collaterology of Chinese Medicine
Introduction
There are many biochemical changes that can interact with each other in diabetic microangiopathine, one of which is the increase of protein glycosylation. Important functional changes are increased blood flow to organs, increased vascular permeability, abnormal blood viscosity, and abnormal platelet and endothelial function. The structural characteristics of diabetic microangiopathosis are thickening of the capillary basement membrane, and it may lead to vascular obliteration and tissue hypoxia and injury. The pathogenesis of diabetic microvascular dysfunction is not fully understood; however, endothelial cell injury and dysfunction and gradual loss of microvascular repair mechanisms are key. In addition, oxidative and hypertonic stress, as well as activation of advanced glycation end products and inflammatory pathways, have been identified as key potential factors.
Hemodynamic Change
Sustained high blood glucose level will lead to damage and destruction of renal cell structure and microvessels in diabetic patients, while abnormal hemodynamics is the early manifestation of diabetic microvascular disorders, it belongs to microcirculation dysfunction and is characterized by increased blood flow and pressure in microvessels. In general, it’s reversible. In the kidney, the microcirculation, such as the glomeruli and peritubular capillaries, supplies oxygen and nutrients to the corresponding areas. Chronic hypoxia is considered to be one of the main mechanisms of renal disease. Impaired blood flow to capillaries leads to reduced oxygen supply to the kidneys, suggesting that reduced microcirculation may contribute to kidney disease [1]. In addition, metabolic and hemodynamic changes that occur in diabetes lead to ultrastructural changes in the glomerular filtration barrier. These structural glomerular changes are associated with increased proteinuria and are markers of generalized systemic vascular dysfunction, and it may represent the common pathogenesis of diabetic renal and extrarenal chronic vascular complications. Peripheral perfusion index (PI) refers to the ratio of non-pulsatile blood flow to pulsatile blood flow in tissue. PI, which represents microcirculatory function, is associated with UAE and eGFR after adjusting for systolic blood pressure and may be a new indicator of DKD in diabetic patients [2]. PDE5 inhibitors (PDE5is) play a protective role in DN to improve perivascular inflammation, improve renal microcirculation, and improve hemodynamic parameters and vascular integrity.
Intrarenal hemodynamic studies have revealed a so-called hemodynamic disorder secondary to glomerular endothelial dysfunction, and it is characterized by preferential contraction of the output arterioles, followed by reduced peritubular capillary flow, and then leading to chronic ischemic injury of the tubule interstitium [3]. The decrease of peritubular capillary flow was negatively correlated with the size of tubulointerstitial fibrosis and decreased gradually with the increase of disease severity from normoalbuminuria to diabetic nephropathy with albuminuria. Therefore, renal microvascular injury has an important impact on the progression of renal disease [4].
Renin-Angiotensin-Aldosterone System
The renin-angiotensin-aldosterone system (RAAS) is an important system involved in hemodynamic changes in diabetic nephropathy. It directly regulates the body’s blood pressure and fluid volume and is involved in blood vessel damage and inflammation. High glucose induces the activation of local renal (tubular epithelial cells, mesangial cells, podiocytes) RAAS, and it increases the production of Ang II and subsequently causes the contraction of bulbar arterioles, leading to higher glomerular capillary pressure. Angiotensin converting enzyme inhibitors and angiotensin receptor blockers can reduce creatinine and proteinuria and slow the progression of nephropathy [5]. In addition, local tissue production of Ang II can activate a variety of intracellular signaling pathways, and also cause inflammation, kidney cell growth, mitosis, apoptosis, migration, and differentiation.
Endothelin 1
In addition to AngII, another effective bulbous arteriole
vasoconstrictor is endothelin 1. It has different functions in kidney :
a) It can simulate RAAS to regulate vasoconstriction, so it
plays an important role in hypertension, endothelial dysfunction,
inflammation and fibrosis.
b) Increased expression of endothelin 1 can activates
signaling cascades, leading to mesangial hyperplasia and
hypertrophy and extracellular matrix production.
c) Endothelin 1 can also activate corresponding receptors,
leading to increased glomerular permeability, worsening
proteinuria and progression of diabetic nephropathy [6].
Oxidative Stress
Oxidative stress (OS) is a key factor in the development and progression of diabetes mellitus and its related complications [7] and the mechanism of oxidative stress induced renal injury has been widely recognized, including the accumulation of late glycation end products [8]. The increased production of oxidative stress is induced by a persistent hyperglycemic state capable of oxidative damage to macromolecules (lipids, carbohydrates, proteins, and nucleic acids). OS is conducive to the generation of oxidative damage of doublestranded DNA histones, and affects the expression of DNA repair enzymes, eventually, the cells die by apoptosis [8]. OS can lead to podal cell injury, endothelial cell dysfunction, mesangial cell injury, elevated level of transforming growth factor -β, microalbuminuria and glomerular apoptosis, and accelerate the progression of DN, leading to end-stage renal disease [9]. Reactive oxygen species (ROS) are produced by aerobic metabolism, and mitochondria are the main source of ROS overproduction, whose capacity exceeds the production of endogenous antioxidants. The NADPH oxidase (NOX) family is a major source of ROS in blood vessels and regulates renal perfusion. It significantly affects the pathological signals of blood vessels [10], renal cortex [11] and medulla [12]. Oxidative stress promotes vascular diseases by promoting vascular smooth muscle proliferation, monocyte/macrophage infiltration, vascular tone changes and matrix metalloproteinase activation [13]. Therefore, oxidative stress induces vascular remodeling and increases preglomerular resistance, it is a key mechanism in the pathogenesis of kidney and cardiovascular disease [14,15].
The hexosamine biosynthetic signaling pathway (HBP) aggravates hyperglycemia-induced diabetic complications by reducing NO production and promoting the possible transcription of some tissue growth factors (TGF-α and TGF-β1). HBP enhancement impairs endothelial function because the O-Glcnacylation inhibits the phosphorylation of Akt/eNOS in endothelial cells and resulting in reduced NO production. Therefore, inhibition of HBP may be a potential intervention target for diabetic vascular complications [16]. Activation of PKC can induce ROS overproduction. ROS, in turn, oxidizes cysteine residues of specific PKC subtypes, leading to activation of PKC and downstream signaling pathways associated with diabetes complications [17]. Therefore, PKC redox modification may play a key role in the onset and development of diabetes complications, and targeted PKC redox modification has great potential in the treatment of diabetes.
Mitochondrial dysfunction plays an important role in the vascular complications of diabetes, especially those related to oxidative stress, dynamic and biosynthesis abnormalities, and autophagy [18]. The increase of mitochondrial fission can affect endothelial function through the increase of ROS [19]. Mitochondrial ROS plays an important role in DM complications including DN [20]. Target cells include glomerular mesangial cells, retinal capillary endothelial cells and nerve cells, which cannot fully regulate intracellular glucose concentration in the environment of diabetes [21]. As a result, these cells are subject to ROS mediated extraordinary OS [21]. In addition, oxidative stress mediated by mitochondrial recombination plays a key role in the development of diabetes complications by activating PKC and increasing the formation of AGEs, which in turn leads to metabolic abnormalities. Therefore, understanding the basic mechanisms of mitochondrial function regulation is crucial to validate new targets for the prevention of vascular complications [22].
Changes in Glomerular Filtration Barrier
The glomerular filtration barrier (GFB) is a complex structure consisting of endothelial cells, a broad basement membrane, and an intercellular junction slit septum that connects the foot processes derived from adjacent podocytes [23]. As blood flows through the glomerulus, the plasma filtrate first passes through the endothelial fenestra and the red blood cells are trapped inside the blood vessels. The basal membrane of the glomeruli acts as a coarse filter barrier for plasma macromolecular proteins, and the hiatus membrane acts as a precise ultrafiltration device that effectively retains albumin and its larger molecular weight in the blood. Endothelial cells composed of endodermis calyx are an important part of glomerular filtration barrier [24]. Diabetes affects the endothelial function of macrovessels and microvessels. Endothelial cell dysfunction in the macrocirculation of diabetes is characterized by decreased bioavailability of nitric oxide, increased oxidative stress, enhanced inflammatory response, up-regulation of adhesion molecules, and destruction of endothelial barrier and abnormal angiogenesis. Angiogenesis is a feature of diabetic microcirculation [25-28]. The glomerular endothelial cell (GEnC) pore is a podocyte - like filter slit, and its special function is a prerequisite for filtering through the glomerular capillary wall. The glomerular filtration rate depends on the aperture area. Vascular endothelial growth factor (VEGF) is a key inducer of pore formation [29].
Inflammatory Cytokines
Tesch [30] confirmed that abnormal inflammatory response plays a key role in the pathogenesis of DN. Inflammatory cytokines, mainly IL-1, IL-6, and IL-18, as well as TNF-α, participate in the occurrence and development of diabetic nephropathy [31]. Inflammatory factors can affect glomerular function by changing renal vascular blood flow and vasoconstriction, regulating extracellular matrix dynamics, endothelial cells and vascular proliferation [32]. Inhibiting the entry of inflammatory cells into the kidney has been shown to have a protective effect on experimental diabetic nephropathy. PI3K signaling pathway plays an important regulatory role in the inflammatory response of DN [33-35]. In addition, the signaling pathway of diabetic microangiopathy is partially cross-linked with the platelet NF-κB pathway, and platelets can release a variety of chemokines and aggravate vascular endothelial cell injury, suggesting that platelets may play a key role in the pathogenesis of diabetic microangiopathy [36].
Metabolic Disorders
Metabolic disorders affect the pathogenesis of diabetic nephropathy, including high glucose, polyol pathway activation, protein kinase C pathway activation, advanced glycation end products (AGE) and so on. Protein kinase C (PKC) is a widely expressed enzyme involved in a wide range of intracellular signal transduction. Its activity is upregulated in the vascular tissues of diabetics, including the aorta, glomerulus, and retina. PKC is involved in the activation and synthesis of MAPK, vascular endothelial growth factor (VEGF) and transforming growth factor β (TGF-β). And NO is released by activating iNOS. Its regulation of vascular endothelial growth factor is associated with abnormal renal blood flow, capillary permeability, and the development of microproteinuria [37]. PKC also plays a key role in hyperglycemiainduced retinal vascular permeability dysfunction and VEGF expression. By inhibiting PKC and thus ROS production, dead cells and pro-inflammatory cytokines are down-regulated. On the contrary, under the continuous high glucose state, PKC is activated, and more DG is generated in the body. In this way, ROS synthesis is increased, and the structure of glomerular basement membrane is changed, and ultimately leads to increased glomerular capillary permeability and accelerated glomerular injury. Therefore, the activation of PKC pathway is also an important factor leading to the occurrence and development of DN [38].
Mechanism of RAS System Activation in Diabetic Nephropathy
Activation of the renin-angiotensin system W(RAS) is involved in the progression of renal damage, including DN. Evidence suggests that RAS overactivity is involved in endogenous pathological changes in atherosclerosis, hypertension and diabetes [39]. RAS components are upregulated, and RAS blockade has beneficial effects. Components of intrarenal RAS are selectively activated in human diabetic kidneys. Interstitial cell infiltration and reninangiotensin activation occur simultaneously, and Ang II may lead to the progression of renal injury in human DN through activation of NFκB pathway and up-regulation of related genes (mainly in renal tubular cells) [40]. Ang II is a cytokine mediated by NFκB and related genes such as cytokines, adhesion molecules and chemokines McP- 1 and RANTES41 [42]. Hyperglycemia increases tissue angiotensin II (Ang II), leading to oxidative stress, endothelial damage, and pathologies of disease, including vasoconstriction, thrombosis, inflammation, and remodeling. Recent studies have found that local RAS in the kidney is independent of systemic RAS [43]. Systemic RAS activity has been reported to be normal or inhibited in diabetes mellitus (DM) [44, 45]. Entosis and absorption of prorenin and angiotensinogen in the kidney and vascular system of DM rats contributes to the increase of RAS and angiotensinogen pressure boosting response [46].
The activation of renal angiotensin system (RAS) plays an important role in the development of DN. Increased RAS activity in local tissues leads to insulin resistance, thereby promoting the progression of DKD. RAS overexpression can induce oxidative stress, it is considered to be the main cause of organ damage in diabetes [47,48]. RAS central block inhibits central oxidative stress and sympathetic nervous activity, leading to RAS activity and oxidative stress reduction in kidney. Tempol central administration reduces brain RAS, thereby reducing renal RAS activity and oxidative stress, so it is suggesting an interaction between kidney and brain RAS/ REACTIVE oxygen, promoting the progression of diabetic nephropathy [49].
TCM Collateral Diseases Treatment Scheme
The theory of collateral diseases originated from Huangdi Neijing, it has been developing in recent years and has made great achievements in clinical practice. Chinese patent medicine developed under the guidance of collateral disease theory has also made great achievements in other fields. Such as: Tongxinluo capsule, Qiliqiangxin capsule and other tongluo drugs, has a unique advantage in the prevention and treatment of major cardiovascular and cerebrovascular diseases; Lianhua Qingwen Capsule is approved for mild, common form of COVID-19 treatment. Therefore, the application of collateral pathology theory to the study of diabetic vascular diseases should also be paid attention to by academic circles.
Baoshenfang
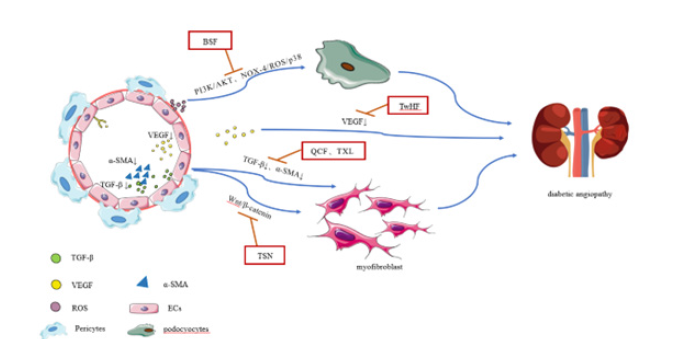
Microalbuminuria is a typical clinical symptom in the early stage of DN, and microalbuminuria can lead to renal insufficiency and rapid progression of DN [50]. Therefore, reducing urinary protein excretion has always been an important treatment strategy for DN [51]. Podocyte injury is the main cause of DN proteinuria [52]. Baoshen Prescription (BSF) is a traditional Chinese medicine compound that promotes blood circulation and drews collateral channels, and is composed of Astragalus membranaceus, Rehmannia Fructus ligustri lucidi, Salvia miltiorrhiza bunge, leeches, scorpions and other Chinese herbs. BSF can reduce proteinuria in DN patients and protect podocyocytes from injury by inhibiting NOX-4/ROS/ P38 pathway [53]. In addition, BSF can reduce proteinuria and podocellular apoptosis in DN patients and delay the progression of DN by regulating the ROS-mediated PI3K/AKT pathway [54].
Tang-Shen-Ning
Tangshenning (TSN) is made up of Chinese herbal medicines, including Astragalus membranaceus, Rehmannia glutinosa Libosch, Rheum palmatum L, Euryale ferox Salisb. ex DC, Cornus officinalis, and Ligusticum chuanxiong Hort. It is a safe and effective drug to treat early DN and improve the inflammation and oxidative stress state of DN. TSN, by activating the Wnt/β-catenin pathway, reverses podocellular epithelial-mesenchymal transformation (EMT), reduces the expression of fibroblast specific protein 1 (FSP- 1) and type I collagen, and alleviates renal injury in DN mice [55]. In addition, TSN can reduce renal fibrosis in DN by regulating the levels of GJA1, CTGF, MMP7 and CCL5 [56].
Tongxinluo
Tongxinluo (TXL) is a traditional Chinese medicine compound composed of 12 kinds of Chinese herbs such as Panax ginseng, centipede woodlouse, scorpio, cicada skin, leech. TXL reduces atherosclerotic lesions by reducing oxidative stress and inflammation in microvascular endothelial cells [57]. In addition, the renal structure and function of DN patients can be improved by regulating the transformation of epithelial cells into stromal cells and protecting podocytes from damage [58]. It was found that TXL can inhibit the secretion of exogenous GECs containing TGF-β1, thus preventing TGF-β1 from transferring to GMCs through exogenous GECs, which may be one of the mechanisms of tongxinluo therapy for DN [59].
Quwan Chencuo Formula
Quwan Chencuo method is the “Huangdi’s Internal Classic Plain Question “(Huang Di, Nei Jing, Su Wen) in the first proposed treatment of edema. Quwan Chencuo Formula is to follow the Quwan Chencuo method into traditional Chinese medicine compound. It is composed of Salvia miltiorrhiza Bunge, cooked Radix et Rhizoma Rhei, Cortex Moutan, Semen Persicae, Radix Puerariae, Poria cocos, Wolf, Semen Plantginis Asi-aticae and Arecae Pericarpium. QCF not only attacks water evil, but also removes blood stasis and dredges collaterals. Studies have found that QCF can reduce the serum fibrosis index of patients with chronic renal insufficiency, such as serum human laminin (LN) and serum type ⅳ collagen (CL-ⅳ). In addition, the mechanism of QCF inhibiting renal fibrosis may be related to up-regulating the expression of E-cadherin and downregulating the expression of TGFβ1 and α-SMA in renal tissuesi [60].
Tripterygium
(TwHF) is a famous Chinese medicine for the treatment of DN, which plays a significant role in reducing proteinuria excretion. Clinical evidence suggests that TwHF combined with ARB/ ACEI is more effective than ARB/ACEI monotherapy in reducing proteinuria in DN patients [61,62]. TwHF has been shown to have a wide range of biological activities, including anti-inflammatory, immunosuppressive, antioxidant and anti-fibrosis, which may be attributed to its renal protective effect on DN [63]. The key mechanism of TwHF against DN may involve reducing renal inflammation by down-regulating VEGFA [64] (Figure).
Currently, diabetes is the single leading cause of end-stage renal disease (ESRD) in the Western world and the leading cause of patients requiring kidney replacement therapy worldwide. Diabetic nephropathy (DN) is a pathological and frightening complication of diabetes, and this microvascular complication affects 45% of patients with T1DM and T2DM. Despite significant advances in our knowledge base today, DN remains a major clinical challenge and burden for healthcare worldwide in the 21st century. Therefore, it is urgent to further understand the pathogenesis of DN and cooperate with the treatment plan of TCM collateral diseases, so as to prevent the progression of the disease and promote the development of new, effective and efficient treatment strategies for this disease.
References
- Choi YJ, S Chakraborty, V Nguyen, C Nguyen, Kim BK, et al. (2000) Peritubular capillary loss is associated with chronic tubulointerstitial injury in human kidney: altered expression of vascular endothelial growth factor. Hum Pathol 31(12): 1491-1497.
- Hiroshi Okada, Muhei Tanaka, Takashi Yasuda, Yuki Okada, Hisahiro Norikae, et al. (2020) Decreased microcirculatory function measured by perfusion index is a novel indicator of diabetic kidney disease in patients with type 2 diabetes. J Diabetes Investig 11(3): 681-687.
- D Verzola, Gandolfo MT, F Ferrario, Rastaldi MP, B Villaggio, et al. (2007) Apoptosis in the kidneys of patients with type II diabetic nephropathy. Kidney Int 72(10): 1262-1272.
- Narisa Futrakul, Punnee Butthep, Prasit Futrakul (2008) Altered vascular homeostasis in chronic kidney disease. Clin Hemorheol Microcirc 38(3): 201-207.
- Radica Z Alicic, Michele T Rooney, Katherine R Tuttle (2017) Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin J Am Soc Nephrol 12(12): 2032-2045.
- Kerstin Benz, Kerstin Amann (2011) Endothelin in diabetic renal disease. Contrib Nephrol 172: 139-148.
- Francesco Prattichizzo, Valeria De Nigris, Elettra Mancuso, Rosangela Spiga, Angelica Giuliani, et al. (2018) Short-term sustained hyperglycaemia fosters an archetypal senescence-associated secretory phenotype in endothelial cells and macrophages. Redox Biol 15: 170-181.
- Sonia Sifuentes Franco, Diego Enrique Padilla Tejeda, Sandra Carrillo Ibarra, Alejandra Guillermina Miranda Díaz (2018) Oxidative Stress, Apoptosis, and Mitochondrial Function in Diabetic Nephropathy. Int J Endocrinol 2018: 1875870.
- Dhruv K Singh, Peter Winocour, Ken Farrington (2011) Oxidative stress in early diabetic nephropathy: fueling the fire. Nat Rev Endocrinol 7(3): 176-184.
- S Rajagopalan, S Kurz, T Münzel, M Tarpey, Freeman BA, et al. (1996) Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest 97(8): 1916-1923.
- W J Welch, C S Wilcox (2001) AT1 receptor antagonist combats oxidative stress and restores nitric oxide signaling in the SHR. Kidney Int 59(4): 1257-1263.
- Zou AP, N Li, Cowley AW (2001) Production and actions of superoxide in the renal medulla. Hypertension 37(2 Pt 2): 547-553.
- Griendling KK, M Ushio Fukai (1997) NADH/NADPH Oxidase and Vascular Function. Trends Cardiovasc Med 7(8): 301-307.
- Bernard Lassègue, Roza E Clempus (2022) Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol 285(2): R277-R297.
- Karen Bedard, Karl Heinz Krause (2005) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87(1): 245-313.
- Pengju Zhang, Tao Li, Xingyun Wu, Edouard C Nice, Canhua Huang, et al. (2020) Oxidative stress and diabetes: antioxidative strategies. Front Med 14(5): 583-600.
- Vicki Thallas Bonke, Jay C Jha, Stephen P Gray, David Barit, Hermann Haller, et al. (2014) Nox-4 deletion reduces oxidative stress and injury by PKC-α-associated mechanisms in diabetic nephropathy. Physiol Rep 2(11):
- Hong Xiang, Ruipeng Song, Jie Ouyang, Ruifang Zhu, Zhihao Shu, et al. (2021) Organelle dynamics of endothelial mitochondria in diabetic angiopathy. Eur J Pharmacol 895:173865.
- Sherene M Shenouda, Michael E Widlansky, Kai Chen, Guoquan Xu, Monika Holbrook, et al. (2011) Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation 124(4): 444-453.
- Inah Hwang, Jiyoun Lee, Joo Young Huh, Jehyun Park, Hi Bahl Lee, et al. (2012) Catalase deficiency accelerates diabetic renal injury through peroxisomal dysfunction. Diabetes 61(3): 728-738.
- Mogensen CE, Christensen CK, Vittinghus E (1983) The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes 32(Suppl 2): 64-78.
- Sungmi Park, Hyeon Ji Kang, Jae Han Jeon, Min Ji Kim, In Kyu Lee, et al. (2019) Recent advances in the pathogenesis of microvascular complications in diabetes. Arch Pharm Res 42(3): 252-262.
- Börje Haraldsson, Jenny Nyström, William M Deen (2008) Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev 88(2): 451-487.
- Marie Jeansson, Karin Björck, Olav Tenstad, Börje Haraldsson, et al. (2009) Adriamycin alters glomerular endothelium to induce proteinuria. J Am Soc Nephrol 20(1): 114-122.
- Yi Shi, Paul M Vanhoutte (2017) Macro- and microvascular endothelial dysfunction in diabetes. J Diabetes 9(5): 434-449.
- Shi Y, Vanhoutte PM (2009) Reactive oxygen-derived free radicals are key to the endothelial dysfunction of diabetes. J Diabetes 1(3): 151-162.
- Paul M Vanhoutte, Yingzi Zhao, Aimin Xu, Susan WS Leung (2016) Thirty Years of Saying NO: Sources, Fate, Actions, and Misfortunes of the Endothelium-Derived Vasodilator Mediator. Circ Res 119(2): 375-396.
- Zhao Y, Vanhoutte PM, Leung SW (2015) Vascular nitric oxide: Beyond eNOS. J Pharmacol Sci 129(2): 83-94.
- Simon C Satchell, Filip Braet (2009) Glomerular endothelial cell fenestrations: an integral component of the glomerular filtration barrier. Am J Physiol Renal Physiol 296(5): F947-F956.
- Tesch GH (2017) Diabetic nephropathy-is this an immune disorder? Clin Sci (Lond)131(16): 2183-2199.
- Juan F Navarro González, Carmen Mora Fernández (2008) The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol 19(3): 433-442.
- Turkmen K (2017) Inflammation, oxidative stress, apoptosis, and autophagy in diabetes mellitus and diabetic kidney disease: the Four Horsemen of the Apocalypse. Int Urol Nephrol 49(5): 837-844.
- Guodong Huang, Jianzhen Lv, Tongyu Li, Guoli Huai, Xiang Li, et al. (2016) Notoginsenoside R1 ameliorates podocyte injury in rats with diabetic nephropathy by activating the PI3K/Akt signaling pathway. Int J Mol Med 38(4): 1179-1189.
- Jin Ni Hong, Wei Wei Li, Lin Lin Wang, Hao Guo, Yong Jiang, et al. (2017) Jiangtang decoction ameliorate diabetic nephropathy through the regulation of PI3K/Akt-mediated NF-κB pathways in KK-Ay mice. Chin Med 12:13.
- Haifeng Liu, Xiaohua Wang, Shengfeng Liu, Hongzhi Li, Xiaohuan Yuan, et al. (2016) Effects and mechanism of miR-23b on glucose-mediated epithelial-to-mesenchymal transition in diabetic nephropathy. Int J Biochem Cell Biol 70:149-160.
- Juanjuan Chen, Wanyu Tan (2020) Platelet activation and immune response in diabetic microangiopathy. Clin Chim Acta 507: 242-247.
- Noh H, King GL (2007) The role of protein kinase C activation in diabetic nephropathy. Kidney Int Suppl (106): S49-53.
- Ighodaro OM (2018) Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed Pharmacother 108: 656-662.
- Zhang X, Lerman LO (2017) The metabolic syndrome and chronic kidney disease. Transl Res 183: 14-25.
- Sergio Mezzano, Alejandra Droguett, M Eugenia Burgos, Leopoldo G Ardiles, Claudio A Flores, et al. (2003) Renin-angiotensin system activation and interstitial inflammation in human diabetic nephropathy. Kidney Int Suppl (86): S64-S70.
- M Ruiz Ortega, O Lorenzo, M Rupérez, V Esteban, S Mezzano, et al. (2001) Renin-angiotensin system and renal damage: emerging data on angiotensin II as a proinflammatory mediator. Contrib Nephrol (135): 123-137.
- Marta Ruiz Ortega, Mónica Ruperez, Oscar Lorenzo, Vanesa Esteban, Julia Blanco, et al. (2002) Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl (82): S12-S22.
- Bader M (2010) Tissue renin-angiotensin-aldosterone systems: Targets for pharmacological therapy. Annu Rev Pharmacol Toxicol 50: 439-465.
- S Anderson, Jung FF, Ingelfinger JR (1993) Renal renin-angiotensin system in diabetes: functional, immunohistochemical, and molecular biological correlations. Am J Physiol 265(4 Pt 2): F477-F486.
- Price DA, Porter LE, M Gordon, Fisher ND, JM De Oliveira, et al. (1999) The paradox of the low-renin state in diabetic nephropathy. J Am Soc Nephrol 10(11): 2382-2391.
- Akihiro Tojo, Satoshi Kinugasa, Toshiro Fujita, Christopher S Wilcox (2016) A local renal renin-angiotensin system activation via renal uptake of prorenin and angiotensinogen in diabetic rats. Diabetes Metab Syndr Obes 9: 1-10.
- Ping Chen, Austin M Guo, Paul A Edwards, Gary Trick, A Guillermo Scicli (2007) Role of NADPH oxidase and ANG II in diabetes-induced retinal leukostasis. Am J Physiol Regul Integr Comp Physiol 293(4): R1619-R1629.
- Maristela Lika Onozato, Akihiro Tojo, Atsuo Goto, Toshiro Fujita, Christopher S Wilcox (2002) Oxidative stress and nitric oxide synthase in rat diabetic nephropathy: effects of ACEI and ARB. Kidney Int 61(1): 186-194.
- Yufeng Liu, Lanying Li, Minzi Qiu, Lishan Tan, Mengbi Zhang, et al. (2019) Renal and cerebral RAS interaction contributes to diabetic kidney disease. Am J Transl Res 11(5): 2925-2939.
- Hermann Pavenstädt, Wilhelm Kriz, Matthias Kretzler (2003) Cell biology of the glomerular podocyte. Physiol Rev 83(1): 253-307.
- Palmer BF (2008) Supratherapeutic doses of angiotensin receptor blockers to decrease proteinuria in patients with chronic kidney disease. Am J Nephrol 28(3):381-390.
- Fangqiang Cui, Dawei Zou, Yanbin Gao, Na Zhang, Jinyang Wang, et al. (2015) Effect of Tongxinluo on Nephrin Expression via Inhibition of Notch1/Snail Pathway in Diabetic Rats. Evid Based Complement Alternat Med 424193.
- Fang Qiang Cui, Long Tang, Yan-Bin Gao, Yue Fen Wang, Yuan Meng et al. (2019) Effect of Baoshenfang Formula on Podocyte Injury via Inhibiting the NOX-4/ROS/p38 Pathway in Diabetic Nephropathy. J Diabetes Res 2981705.
- Fang Qiang Cui, Yue Fen Wang, Yan Bin Gao, Yuan Meng, Zhen Cai, et al. (2019) Effects of BSF on Podocyte Apoptosis via Regulating the ROS-Mediated PI3K/AKT Pathway in DN. J Diabetes Res 2019: 9512406.
- Fang Qiang Cui, Yan Bin Gao, Yue Fen Wang, Yuan Meng, Zhen Cai, et al. (2021) Effect of Tang-Shen-Ning decoction on podocyte epithelial-esenchymal transformation via inhibiting Wnt/beta-catenin pathway in diabetic mice. Ann Palliat Med 10(12): 12921-12936.
- Jiajun Liang, Jiaxin He, Yanbin Gao, Zhiyao Zhu (2021) Exploring the Potential Mechanism of Tang-Shen-Ning Decoction against Diabetic Nephropathy Based on the Combination of Network Pharmacology and Experimental Validation. Evid Based Complement Alternat Med 1025053.
- Xiao Li Wu, Bin Zheng, Li Shuang Jin, Ruo Nan Zhang, Ming He, et al. (2015) Chinese medicine Tongxinluo reduces atherosclerotic lesion by attenuating oxidative stress and inflammation in microvascular endothelial cells. Int J Clin Exp Pathol 8(6): 6323-6333.
- Jin yang Wang, Yan bin Gao, Na Zhang, Da wei Zou, Li ping Xu, et al. (2014) Tongxinluo ameliorates renal structure and function by regulating miR-21-induced epithelial-to-mesenchymal transition in diabetic nephropathy. Am J Physiol Renal Physiol 306(5): F486-F495.
- Xiao Ming Wu, Yan Bin Gao, Li Ping Xu, Da Wei Zou, Zhi Yao Zhu, et al. (2017) Tongxinluo Inhibits Renal Fibrosis in Diabetic Nephropathy: Involvement of the Suppression of Intercellular Transfer of TGF-[Formula: see text]1-Containing Exosomes from GECs to GMCs. Am J Chin Med 45(5): 1075-1092.
- Rui Zhu, Xing Guo Du, Sheng Lan Yang, Yan Ran Wu, Jian Guo Liu, et al. (2019) Effect of Quyu Chencuo Formula () on Renal Fibrosis in Obstructive Nephropathy Rats. Chin J Integr Med 25(3): 190-196.
- Chang Xiong, Li Li, Wang Bo, Huang Chen, Liu Xiao Wei, et al. (2020) Evaluation of the efficacy and safety of TWHF in diabetic nephropathy patients with overt proteinuria and normal eGFR. J Formos Med Assoc 119(3): 685-692.
- Daijin Ren, Chao Zuo, Gaosi Xu (2019) Clinical efficacy and safety of Tripterygium wilfordii Hook in the treatment of diabetic kidney disease stage IV: A meta-analysis of randomized controlled trials. Medicine (Baltimore) 98(11): e14604.
- Wei Jun Huang, Wei Jing Liu, Yong Hua Xiao, Hui Juan Zheng, Yao Xiao, et al. (2020) Tripterygium and its extracts for diabetic nephropathy: Efficacy and pharmacological mechanisms. Biomed Pharmacother 121: 109599.
- Yuyang Wang, Tongtong Liu, Fang Ma, Xiaoguang Lu, Huimin Mao, et al. (2020) A Network Pharmacology-Based Strategy for Unveiling the Mechanisms of Tripterygium Wilfordii Hook F against Diabetic Kidney Disease. J Diabetes Res 2020: 2421631.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.